2023 Ozone Hole Ranked 16th Biggest, According to NASA and NOAA Scientists,

What is an ozone hole? Around the start of spring in the Southern Hemisphere (August–October), a region of highly Antarctica has a depleted stratospheric ozone layer. This is known as the ozone hole, although it is not really a “hole” with no ozone.
What is an Ozone?
The oxygen molecules we inhale daily usually consist of two oxygen atoms (you might have noticed that it is written as “O2” to indicate this). On the other hand, ozone has three oxygen atoms rather than two (O3), even though it is also an oxygen molecule.
Although ozone is extremely minute—consisting of less than 0.001% of the oxygen (O2) in the Earth’s atmosphere—it plays a crucial role in supporting life.
Ozone is produced when two oxygen molecules react with the sun’s ultraviolet (UV) light. This process involves splitting an oxygen molecule, which releases two oxygen atoms that can connect with other oxygen molecules to form ozone trioxide (O3).
The stratosphere is the primary location for ozone production, creating the so-called “ozone layer” since ultraviolet light is more potent at higher altitudes in thinner air. The ozone layer is between 10 and 40 kilometres above ground, with a maximum height of 25 kilometres.
Because it can absorb the most harmful ultraviolet radiation, UV-B, the ozone layer plays a crucial role in protecting Earth’s biota. Its wavelength range is 280–315 nanometers.
A stratospheric temperature inversion occurs when ozone in the stratosphere absorbs ultraviolet radiation, warming the air around it. The accompanying diagram illustrates this phenomenon.
How does an Ozone Hole Develop?
Antarctica has a large ozone hole in its stratosphere around the start of spring in the Southern Hemisphere (August–October). This is known as the ozone hole, although it is not really a “hole” with no ozone.
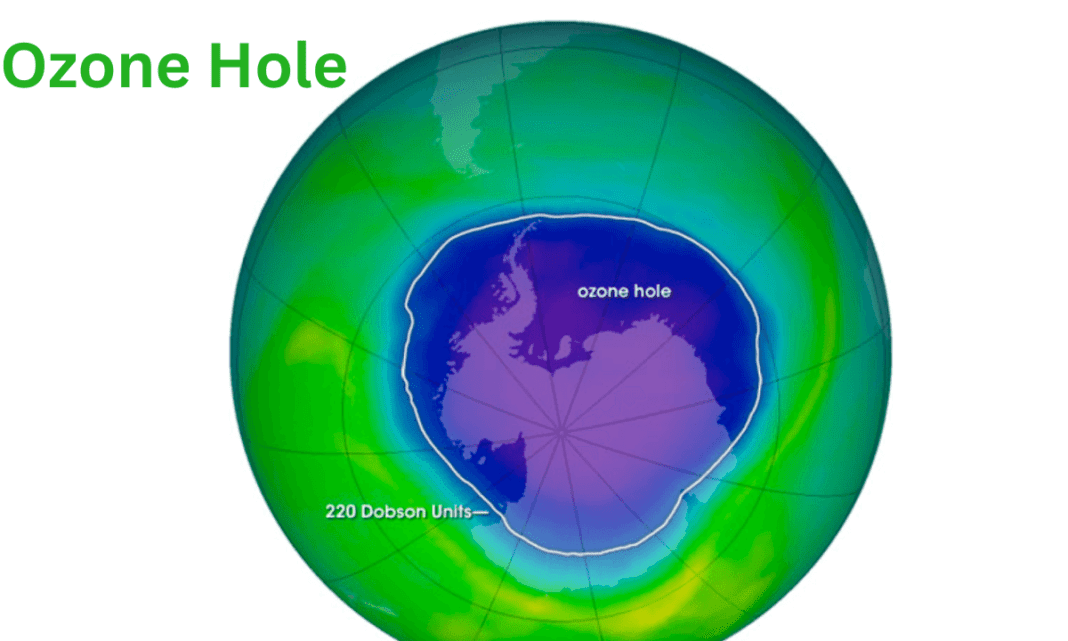
Satellite equipment sends images of the ozone layer over Antarctica daily to Earth. (This image was taken above Antarctica on October 4, 2004.) According to the historical record, the total column ozone of 220 Dobson Units was not recorded before 1979.
An aeroplane field expedition over Antarctica revealed that chlorine and bromine chemicals promote ozone depletion, which is why the area depicting ozone depletion is defined as 220 Dobson Units.

Opaque and Chlorofluorocarbons
The widespread belief that chlorofluorocarbons (CFCs) are to blame for the ozone hole is well-founded. Refrigerant and propellant devices and procedures release CFCs into the air.
Because they are stable at lower altitudes, CFCs can be around for decades. Due to their lengthy lifetime, some CFCs can reach the stratosphere. Stratospheric ultraviolet radiation detaches chlorine atoms (Cl) from chlorofluorocarbon (CFC) molecules.
After dissolving ozone, a chlorine atom undergoes a cascade of chemical events that release the atom back into the atmosphere unaltered, where it can continue to deplete ozone levels.
The less well-known aspect of the tale is that the eventual ozone depletion is not instantaneous, even though chlorine atoms released by CFCs do so.
Two compounds formed from most of the chlorine that escapes CFCs can serve as chlorine reservoirs for the future since they are pretty stable under typical atmospheric circumstances. How then does chlorine drain from the tank every spring?
Food Chains in Peril: How the Melting Ocean Floor is Changing Food Chains
Clouds in The Polar Stratosphere and Ozone
Hydrochloric acid and chlorine nitrate, the two compounds responsible for storing most atmospheric chlorine, are stable when exposed to typical atmospheric conditions. However, air conditions are unusual during the Antarctic winter’s long months of polar darkness.
The air at the centre is isolated by a polar vortex, a never-ending swirl of stratospheric winds. Even though the air in Antarctica is skinny and dry, clouds can still develop due to the frigid air inside the vortex caused by the total darkness of the region.
This is the only area in the atmosphere where specific chemical reactions occur. Depending on the temperature, these peculiar reactions can only happen on the surface of water, ice, or nitric acid particles in the polar stratosphere’s clouds.
These transform the chlorine reservoir’s inert compounds into their active counterparts, most notably chlorine gas (Cl2).
In October, when sunlight reaches the South Pole again, ultraviolet light quickly divides chlorine atoms, releasing free chlorine into the stratosphere. It participates in catalytic reactions that deplete ozone molecules while replenishing chlorine production.
A single chlorine atom can deplete hundreds of ozone molecules through a catalytic process. A significant portion of the ozone depletion is caused by bromine, which is involved in an additional catalytic interaction with chlorine.
Early in the spring, as temperatures rise and the polar vortex begins to weaken, the ozone hole widens, and the air is no longer isolated.
The chlorine species, which deplete the ozone layer, spread as air from lower latitudes mixes with that of the polar region. The ozone layer will return to a stable state by next spring.
The Role of Technology in Modern Family Dynamics
Ozone Hole Ranked 16th Biggest in 2023,


According to NASA and NOAA’s yearly satellite and balloon-based observations, the Antarctic ozone hole in 2023 reached its most considerable extent on September 21. It was the eleventh most excellent single-day ozone hole since 1979, covering 26 million square kilometres or 10 million square miles.
From September 7 to October 13, this year’s ozone hole was the 16th largest. It averaged 8.9 million square miles (23.1 million square kilometres), the size of North America.
Paul Newman, head of NASA’s ozone research team and principal scientist for Earth sciences, expressed his opinion that the ozone hole is relatively small. “Somewhat improved ozone levels this year due to declining levels of human-produced chlorine compounds and assistance from active Antarctic stratospheric weather.”
The ozone layer, which is found in the stratosphere, protects Earth from the sun’s damaging UV rays, much like a natural sunscreen. As the ozone layer depletes, humans are less shielded from ultraviolet radiation that can cause cataracts, skin cancer, sunburns, and other skin conditions.
The Antarctic continent is enveloped in an “ozone hole” that forms every September as the ozone layer gradually diminishes.
The “ozone hole” is a scientific metaphor for the region when ozone concentrations above Antarctica fall significantly below the historical threshold of 220 Dobson Units.
Still, it does not actually contain a vacuum of ozone. Since 1979, when scientists first discovered ozone depletion, they have monitored Antarctic ozone levels annually.
The initial step in losing Antarctic ozone happens when chlorine and bromine-containing man-made substances reach the stratosphere.
The decomposition of these compounds releases chlorine and bromine, triggering chemical processes that deplete ozone.
Aerosol sprays, foams, air conditioners, fire suppressants, and freezers heavily utilise ozone-depleting chemicals, which include chlorofluorocarbons (CFCs). The primary chemicals that deplete the ozone layer, chlorofluorocarbons, have half-lives 50–100 years in the atmosphere.
According to the Montreal Protocol and its subsequent revisions, which went into effect in 1987, all CFC and other ozone-depleting chemical production was to cease by 2010.
The ensuing decrease in emissions has resulted in evidence of stratospheric ozone recovery and a reduction of ozone-destroying compounds in the atmosphere.
Researchers from both agencies monitor the ozone layer using instruments aboard NASA’s Aura, NOAA-NASA Suomi NPP, and NOAA-20 satellites. Chlorine, a known ozone-depleting gas, can also be estimated using Aura’s Microwave Limb Sounder.
By monitoring the ozone concentration within the hole, scientists can also track the average level of depletion.
The thickness of the layer is measured at NOAA’s South Pole Baseline Atmospheric Observatory using a Dobson spectrophotometer on the ground and weather balloons equipped with ozonesondes.
According to NOAA’s readings, the low reading over the South Pole on October 3 was 111 Dobson units (DU). NASA’s observations averaged across a larger region, with a low of 99 DUs on the same date. An average of 225 DU was present above Antarctica in 1979.
“While the total column ozone is never zero, in most years, we will typically see zero ozone at some altitudes within the stratosphere over the South Pole,” stated Bryan Johnson, a research chemist and project head for the Global Monitoring Laboratory’s ozonesonde group, who is affiliated with NOAA.
“Ozone levels in the stratosphere typically drop by nearly 100% annually, but this year we saw a depletion of about 95%.”
This year’s Ozone depletion was probably caused partly by the Hunga Tonga-Hunga Ha’apai volcano, which fiercely erupted in January 2022 and sent a massive plume of water vapour into the stratosphere. Early in the season, such water vapour probably amplified ozone-depletion reactions over Antarctica.
“The ozone hole would likely be smaller this year if Hunga Tonga hadn’t gone off,” Newman added. “Although we are aware that the eruption reached the stratosphere of Antarctica, the extent to which it affected the ozone hole remains uncertain.”
By 2100, Experts Predict That The World Population Will Have Practically Halted.
The Ozone Layer: A Natural Sunscreen for Our Planet


Chlorofluorocarbons (CFCs) were chemicals widely employed in many household items and appliances a few decades ago. In a 1974 publication, chemical researchers Sherwood Rowland and Mario Molina found that CFCs were depleting the ozone layer, Earth’s protective shield from the sun’s UV radiation.
What is The Cause of The Ozone Hole?
The ozone hole formed due to human-caused atmospheric pollution from chlorine—and bromine-containing compounds. The main compounds involved are chlorofluorocarbons, halons, and carbon tetrachloride.
CFCs, in particular, have a long history of usage in several industries, including air conditioning, foam packaging, aerosol spray can production and refrigeration.
These chemicals can harm the ozone layer because they are so inert that they remain in the atmosphere long enough to be carried to the stratosphere.
Specific meteorological conditions are necessary for the complicated mechanisms that cause CFCs and other ozone-depleting compounds to degrade ozone. Here is a simplified explanation of the process that involves CFCs:
When nonreactive CFCs reach the stratosphere, ultraviolet light can decompose them, releasing reactive chlorine.
Clouds in the stratosphere are necessary for this to happen because they provide surfaces for ice-crystal interactions.
After escaping from CFCs, chlorine (Cl) interacts with ozone (O3) to produce ClO and O2. ClO rapidly decomposes to release the Cl atom, which can then undergo the reaction again with another molecule of ozone.
This allows one chlorine atom to consume almost 100,000 ozone molecules before escaping the stratosphere.
It is also puzzling that the majority of ozone depletion occurs over Antarctica. The south polar stratosphere is the ideal place for the depletion of the ozone layer. However, CFCs and other ozone-depleting chemicals can originate from anywhere.
In the austral winter and early spring, the determining factor is the absence of atmospheric mixing between the south polar latitudes and air from elsewhere, along with the existence of stratospheric clouds.
Eco-Anxiety: How Can We Bring Piece In This Changing World
Both The Issue and Potential Remedies
The ozone layer is like a superhero that protects our planet from the lousy UV-B rays.” which are known to cause cancer and hinder plant development. Marine life, particularly phytoplankton, is vulnerable to UV radiation since it can reach the ocean’s surface.
It is obviously in our interest to ensure that we do not harm the ozone layer since its absence would hinder photosynthesis by plants, and ecosystems would not be able to function as they do today.
The world community quickly agreed on a course of action in the 1980s when the extent of ozone depletion over Antarctica became clear. Rapid governmental intervention prevented the atmospheric buildup of ozone-depleting chemicals, which would have widened the Antarctic ozone hole and reduced atmospheric ozone levels elsewhere.
The Montreal Protocol of 1987 aimed to eliminate the use of ozone-depleting chemicals. The Protocol entered into force in 1989. In addition, it encourages studies to find ozone-safe alternatives to CFCs and similar compounds.
One of the most successful international treaties, it has since undergone multiple revisions and 195 ratifications.
The treaty reduced ozone-depleting emissions worldwide. However, the ozone layer will not completely regenerate until around 2050 because many of these chemicals have a lengthy atmospheric residence time (for instance, CFC-12 stays in the atmosphere for about 100 years).
The Ozone Hole and Its Measurement in The Stratosphere


In Dobson units, ozone is the total amount in an atmospheric column. If ozone were to build a layer 0.01 mm thick at average sea-level pressure and temperature, one Dobson unit would represent the amount of ozone present.
According to an average Dobson reading of 300 Dobson units, the ozone layer would only be around 3 mm thick if transported down to sea level.
The Dobson spectrophotometer gave rise to the moniker “Dobson unit” for this particular set of measurements. It determines the amount of ozone in the sky by observing the ratio of two UV light wavelengths, one more heavily absorbed by ozone than the other.
Halley Research Station has been measuring the ozone layer using this method since 1956. The “Ozone hole was discovered in 1985,” when researchers from the British Antarctic Survey revealed data revealing a dramatic drop in ozone levels over Halley since the 1970s, especially during the austral spring.
After that, ground-based and satellite-based methods of continuously monitoring the ozone hole’s extent began.
Don’t miss out on daily insights! Subscribe now, like and share our content, and leave your comments. Your support is greatly appreciated!